Ionization Mechanisms in UV-MALDI
Table of Contents Next: The CPCD Model
2. Desorption and Ablation
The Different Types of Phase Change in MALDI
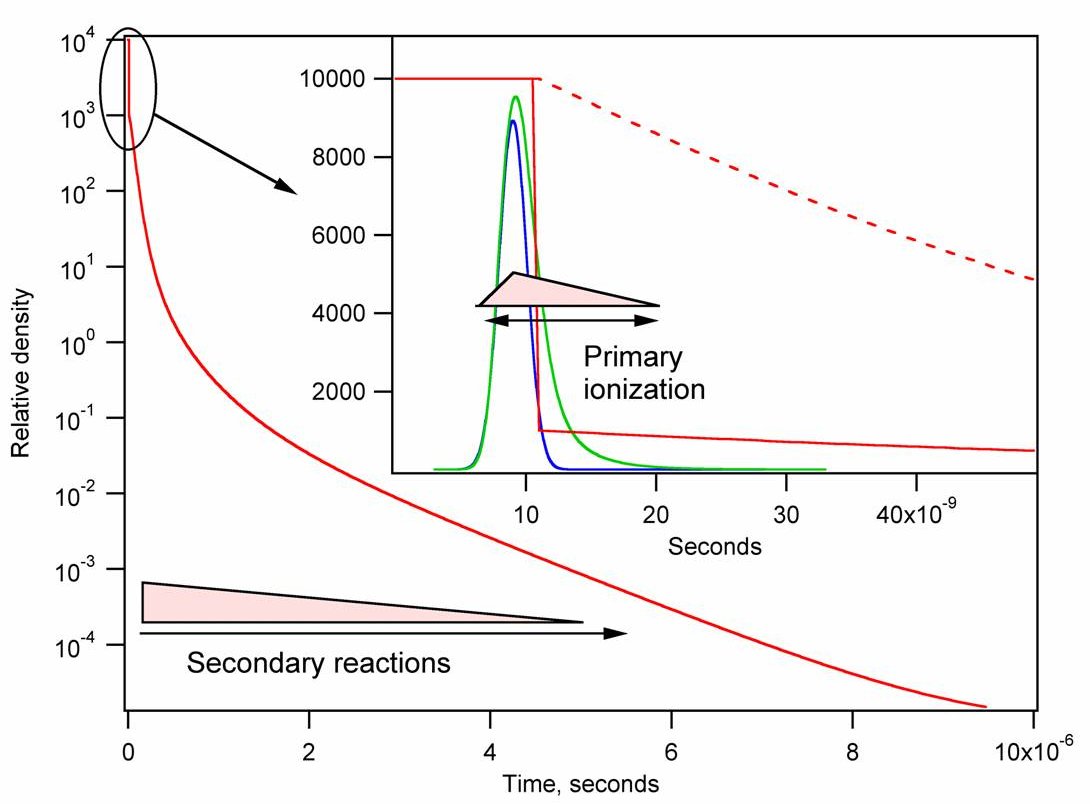
If we want to understand ionization, the rapidly changing environment
of the reactive species must be taken into account. Most important is
the density change and its time scale. The laser pulse typically has a
temporal width of a few nanoseconds, but the time needed for the
resulting vapor to expand to a collision-free density is many
microseconds. This is illustrated in the next figure.

Figure 1. Density change during a MALDI event. The blue curve represents a typical laser pulse, green the population of excited states, and red the relative density of the expanding plume vs. time. The dashed line is for a phase explosion while the full line is for desporption.
Fig. 1 corresponds to a sample that changes entirely from solid to gas. Smooth evaporation from the surface is known as desorption, Fig. 2 and Fig. 3. Although part of the name of the method, this type of phase change is not usually a major contributor to the ejection of material in a typical MALDI event.
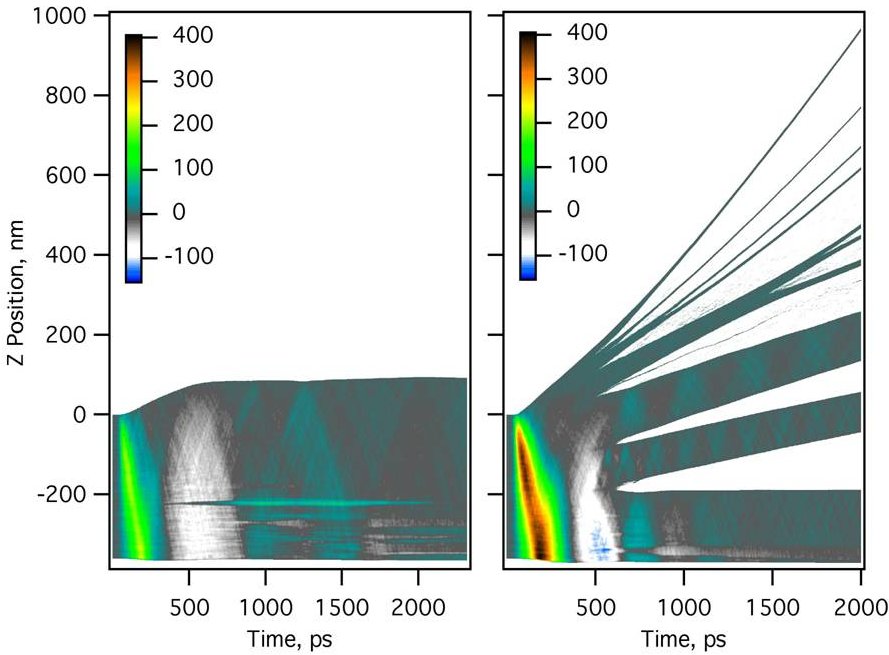
Figure 2. Desorption, spallation and phase explosion in MALDI. Pressure (MPa) vs. time as calculated by molecular dynamics (see Chapter 5) for different laser pulse energies. The lowest energy (left) leads to thermal expansion and smooth evaporation from the top surface. A higher pulse energy (right) leads to phase explosion and ejection of clusters in the upper layers. In addition, the expansive (rebound) pressure wave (white) is strong enough to break the cold material at considerable depth, causing spallation of thick layers. The 355 nm laser pulse width is 35 ps, centered at 40 ps, DHB matrix.
Instead, the rate and depth of laser heating us usually such that subsurface nucleation and gas bubble formation occurs. This results in a phase explosion, Fig. 2 and Fig. 3, which ejects not only gas but also clusters or droplets of condensed material. These may or may not later fully vaporize, depending on their energy content.
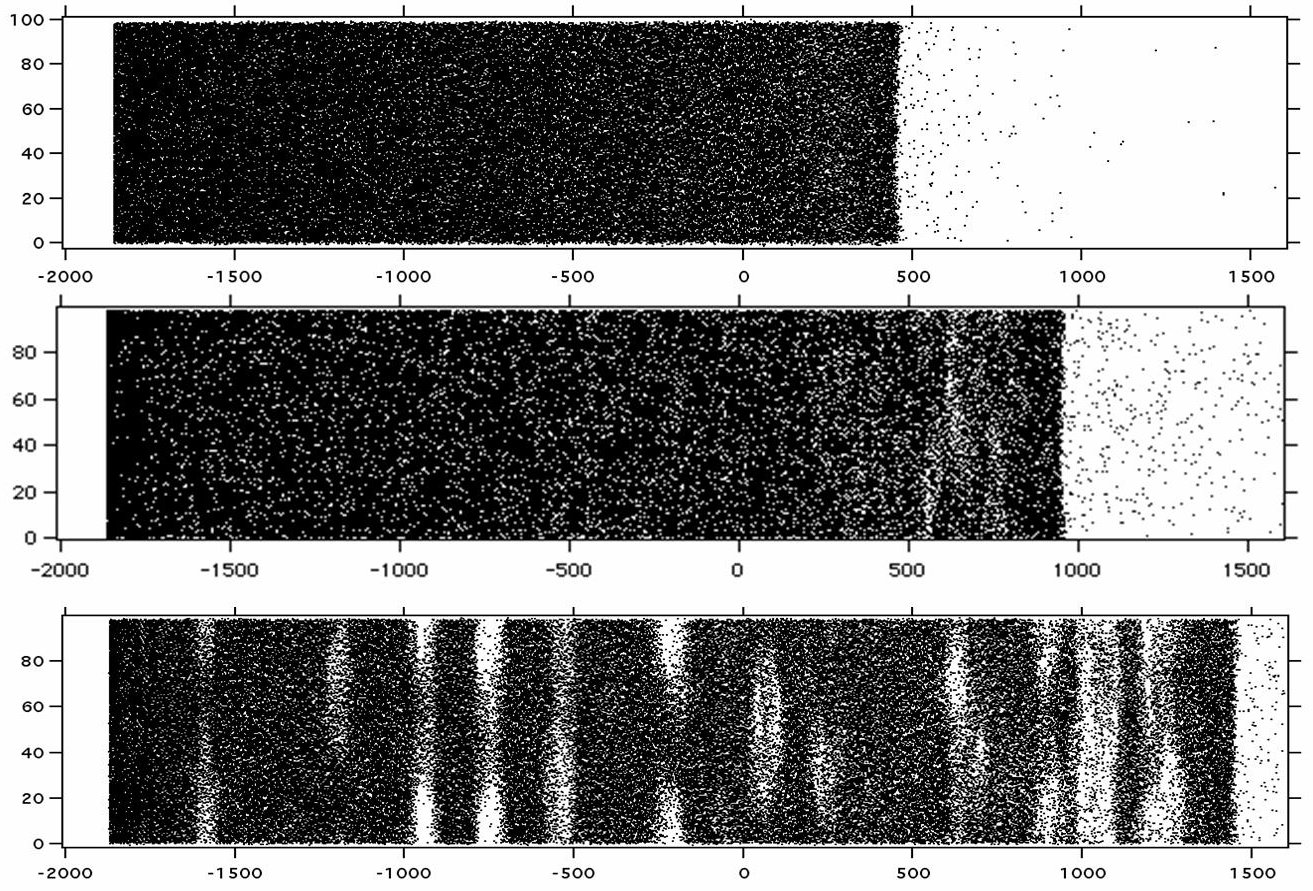
Figure 3. Phase explosion in MALDI. Lateral snapshots of molecular dynamics MALDI simulations vs. time (see Chapter 5). The laser is incident from the right, axes are in Angstrom (0.1 nm). As the material heats and expands, molecules first desorb smoothly from the upper surface (top panel). A bit later (middle panel), subsurface nucleation is beginning. In the lower panel, a frothy mix of droplets and dense vapor extends far below the surface.
The fast laser heating causes a pressure wave to propagate into the material. It is a compression wave followed by an rebound, or expansion wave. If the rebound is sufficiently strong, the material can fracture and leave the surface, a process called spallation, Fig. 2. This results in large, cold clusters, which do not have sufficient energy to fully vaporize over time.
Altogether, the ejected material is known as the MALDI plume. A MALDI event often includes desorbed molecules, followed by the froth of the phase explosion and the chunks of spallation. It is not a simple environment. For analytical purposes, however, we are interested in the free molecules which become ionized. Anything which remains in condensed clusters, droplets or chunks is not relevant for the final mass spectrum. However, those ions which become free start in the condensed phase, so we have to consider everything from solid to high pressure fluid to a rapidly expanding gas and, finally, free molecules.
The MALDI desorption/ablation process has been reviewed by Klaus Dreisewerd, Chem. Rev., vol. 103, p. 295 (2003).
Next: The CPCD Model of UV MALDI Ionization:
MALDI Ionization Tutorial © Copyright 2007-2016 Richard Knochenmuss